HIPAA-Compliant "Safe" & Convenient Telemedicine Consultations
A Hub of Excellence and Innovation in Women's Health
Cutting-Edge Treatments and Compassionate Care at the Forefront of Endometriosis & Gyn Oncology
G.O. Institute
Santa Monica, Marina del Rey & Avila Beach, California
Advanced Robotic Pelvic Surgery & Integrative Healing

Gynecologic Cancer Treatment
Personalized treatment options for endometriosis associated cancers, ovarian cancer and uterine cancer

Advanced Endometriosis Excision
Advanced endometriosis excision, using minimally invasive surgery, even when prior surgery has failed.

Ovarian Cysts &
Pelvic Masses
Pelvic masses like ovarian cysts and tumors
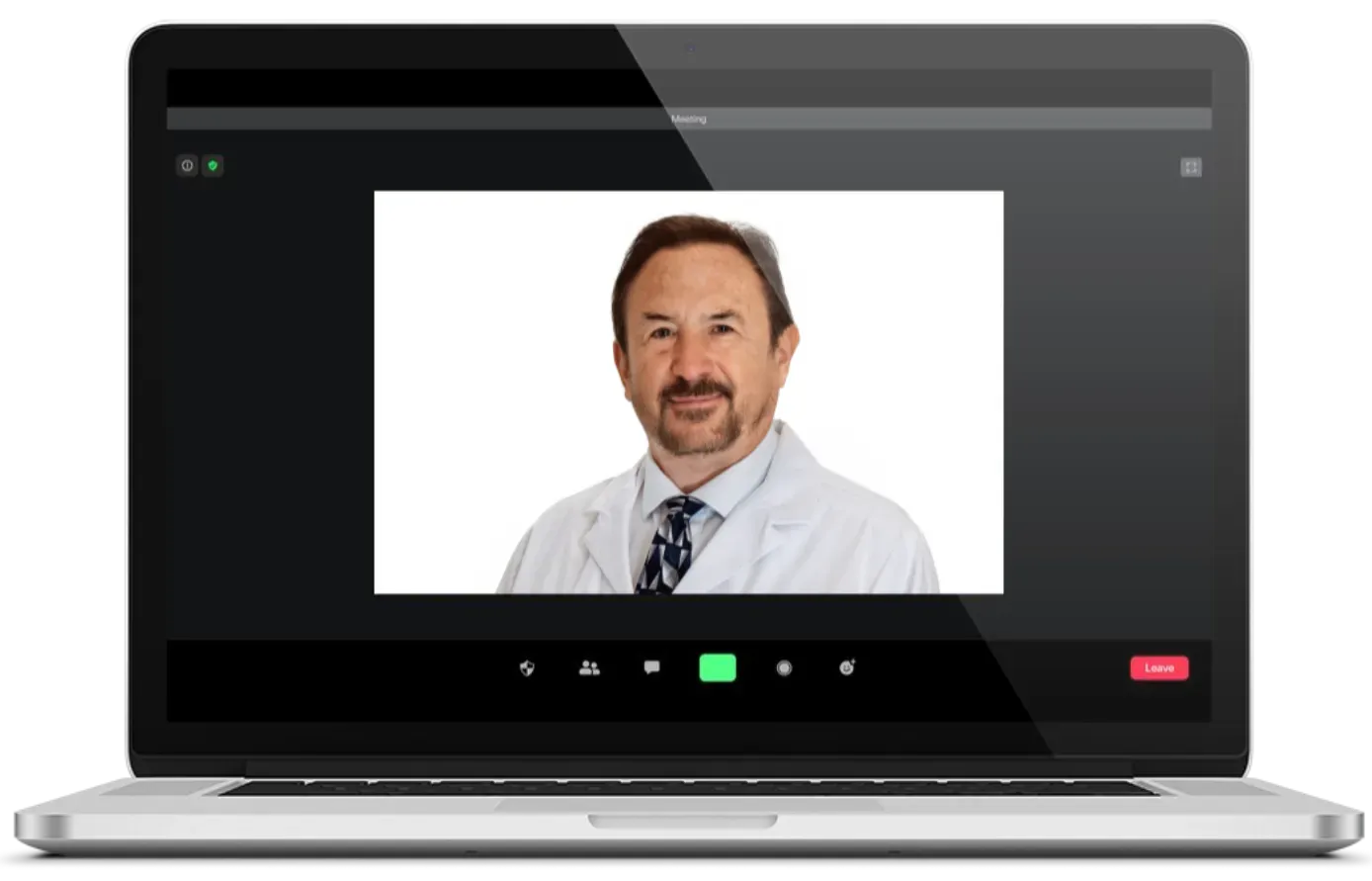
Quickly Schedule a Convenient At-Home Virtual Visit
Don’t get surgery or treatment based on YESTERDAY’S science.
Discover ALL your minimally invasive surgical, precision molecular medical and integrative holistic nutritional options!
Research Informed
21st Century Cutting-Edge Treatment Options
Surgical and medical therapy options are based on the latest best published research information
Individualized Care
Extensively Discussed & Applied to YOU as an Individual
Research studies are focused on hundreds or thousands of patients, so how that is applied to you as an individual is critical to success.
Integrative-Injected
Science-informed Nutrition & Mind-Body
Many "alternative" and "natural" options are useless and harmful but many are very helpful. We help you find what really works!
Hand Guided-Care
Hand-held Guidance Throughout Your Experience
We believe the personal "human touch" to help guide you is crucial for your recovery and focus on making sure you are supported.
Your Health Is A Priority For Us
When facing advanced endometriosis, complex benign surgery or surgical and medical therapy for cancer and pelvic masses, the challenge is not only to beat the disease but to restore health and vitality as quickly as possible! There are many benefits to this approach, including:
-
Home faster after surgery, often the same day or next morning with minimally invasive techniques
-
Less invasive means fewer complications, including less pain, small blood loss, less infection, fewer readmissions
-
Reducing treatment toxicity, using 21st century targeted therapies and holistic support, can be tailored to you by clinical and molecular analysis


Your Quality Of Life Is Too
Because of the benefits of faster surgical recovery and a focus on holistically restoring health, this means you are back with family and loved ones, thriving and enjoying the best that life has to offer
-
Drive & get around sooner, because less need for opioids due to less pain means you’re safe to go anywhere you want quickly
-
Socialize faster, because less pain and less surgical trauma means you can get into life’s routine must faster
-
Get to the next treatment ASAP, if needed in your journey to health. This is absolutely HUGE in order to increase chances for a cure.
-
Thrive during chemo-biotherapy, with integrative holistic support you can maximize quality of life activities during treatment
Meet Your Team
Distinctive Individualized Care Experience
We deliver higher-level limited-availability services by application review only, so when you are accepted as a patient you join us as a VIP and not treated as an impersonal number getting cookie-cutter “ok-level” care
….as we all know, “just OK is not OK” !!
What Our Patients Say
In the end, what people say reflects the age-old saying “the proof is in the pudding”. We walk the talk and help people emerge from the other end as quickly as possible, with personal support.
“Dr. Vasilev is a very caring doctor and that is really important. The whole team is focused on a caring attitude”
Patricia B.
Santa Monica, CA
“Heartfelt gratitude to my magnificent surgeon, Dr. Vasilev, to Lisa and to the entire team.”
Sandra C.
Santa Monica, CA
“Dr Vasilev and his team were absolutely amazing. The robotic surgery was fantastic. ”
Marion W.
Los Angeles, CA



Appointments Extremely Limited
Convenient TeleHealth Consultations
Consultations very limited so we can concentrate on those we can help the most.
Initial infection-safe consult is online with testing and examination as needed.
Knowledge Is Empowering….
Frequently Asked Questions
About Gynecologic Oncology, minimally invasive gynecologic robotic surgery, advanced endometriosis excision surgery, precision cancer therapy, and integrative holistic medicine healing.
© Steven Vasilev MD | Gynecologic Oncology. All Rights Reserved 2015-2024.



